Understanding Police Clearance Police Clearance is an official document issued by...
Read More

.BEYOND BLOGGING
GET STARTED NOWAre You Ready?

Focus on creating a blog , that is great for your readers.
Don't focus on a great blog.
Click HereRecent Posts
The Essentials Hoodie A Symbol
The fashion world is constantly evolving and trends come and...
Read MoreBaagh Enterprises: Your Go-To Dressing
Introduction Baagh Enterprises is a top-tier dressing store, offering a...
Read MorePrescription Safety Eyewear Program Online:
Introduction Protecting employees’ vision is a critical aspect of workplace...
Read MoreUnveil Your Radiance with RJ
At RJ Hair Creations, we believe that confidence starts with...
Read MoreEssential Self-Care for College Students:
Why College Students Need Special Care Products College life comes...
Read MoreBefore the Dates: Making Every
In today’s fast-paced world, it’s easy to lose track of...
Read MoreAchieve Financial Stability With Car
Then again, they have to deal with different challenges like...
Read MoreDesign
A Sneak Peak Into Our Exclusive Articles
Premium content written by highly experienced authors.





Our Categories

Business

Digital marketing

Finance

Review

Technology

Web development
Popular Posts
What To Look For When
Floor sanding is essential for restoring the appearance and integrity...
Read MoreLooking for the Best Plastic
Plastic surgery has become increasingly popular as more people seek...
Read MoreThe Future of Cosmetic Chemicals:
Comprehensive historical analysis of Global Cosmetic Chemicals Market for has thoroughly analyzed...
Read MoreHow to Treat Acne Holes
Acne scars, especially those stubborn holes left behind after a...
Read MoreSpider Tracksuit The Pinnacle of
In the ever-evolving world of athleisure and streetwear, the Spider...
Read MoreHow AI Impacts The Labour
A transformed labor market landscape can be envisioned with the...
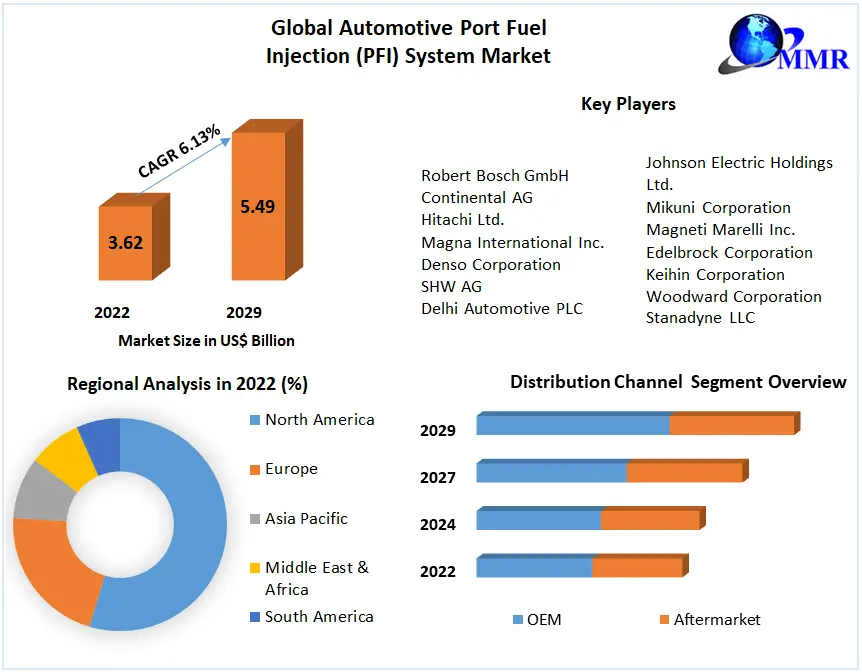
Read MoreAutomotive Port Fuel Injection (PFI)
Automotive Port Fuel Injection (PFI) System Market is expected to...
Read MoreOur Services

Search engine optimization
SEO services optimize websites for better visibility and ranking on search engines to increase organic traffic and improve online presence.

social media optimization
Social media optimization services enhance a company's social media presence through strategies such as content creation, engagement, and audience targeting.

Paid advertisement
Paid advertisement services involve managing and running paid advertising campaigns across various platforms to increase visibility and drive targeted traffic.

web application development
Web application development involves the creation and implementation of interactive software applications that run on web browsers or mobile devices.

LMS development
LMS website development involves the creation of a website specifically designed to host and deliver online educational content through a Learning Management System.

Ecommerce development
Ecommerce website development involves creating an online platform where businesses can sell products or services directly to customers over the internet.

Data Entry
Data entry services are commonly outsourced by businesses to save time and resources, allowing them to focus on core activities. It is essential to maintain.

App development
App development services involve the creation and development of mobile applications for various platforms, such as iOS (iPhone/iPad) and Android devices.

graphic designing
Graphic design services encompass a wide range of creative solutions to visually communicate messages and enhance brand identity.